Abstract
Introduction: Differences in coagulation assay reagents may lead to variable results for some extended-half-life (EHL) recombinant factor VIII (rFVIII) products, with the potential to adversely affect patient care. Laboratories assessing EHL-rFVIII activity can choose from many available one-stage assay and chromogenic assay reagents. A field study was conducted to evaluate the ability of clinical laboratories to accurately measure FVIII activity in plasma samples spiked with BAY 94-9027, an EHL rFVIII, when using diverse assay reagents and when guided in the choice of reagent. In a regional subanalysis, BAY 94-9027 field study results from clinical laboratories in the United States and Canada were compared with those from Europe and Israel.
Methods: In this 2-part study, a broad range of laboratories in the United States, Canada, Europe (Austria, Germany, Italy, Romania, Spain, Switzerland, United Kingdom), and Israel were provided samples containing defined concentrations of BAY 94-9027 or an unmodified rFVIII (antihemophilic factor [recombinant] plasma/albumin-free method [rAHF-PFM]) as a control. In part 1, each laboratory measured FVIII activity using their routine methods (one-stage assay, chromogenic assay, or both); laboratories that had ≥2 routine one-stage assays were asked to perform sample testing with one of the less commonly used assays in the laboratory to ensure that part 1 captured both the prevalence and heterogeneity of one-stage assays used in the geographic regions studied. In part 2, laboratories used one-stage assays with SynthASil and Pathromtin SL reagents provided by Bayer; SynthASil and Pathromtin SL have previously been shown to accurately measure BAY 94-9027 and full-length rFVIII products. The field study data were analyzed to identify the most commonly used reagents in the 2 regions (United States/Canada and Europe/Israel) and to assess if regional differences resulted in differences in accuracy of FVIII activity measurement.
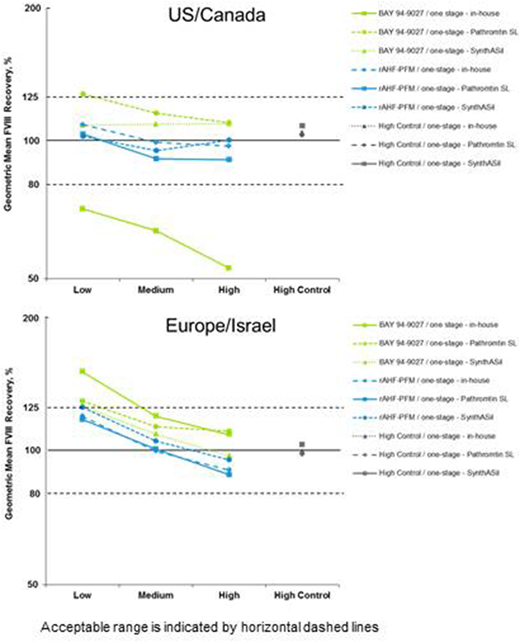
Results: 52 laboratories (US/Canada, n=25; Europe/Israel, n=27) participated in the field study. The one-stage reagent SynthASil was commonly used in all countries (n=15) in part 1, but differences were seen in the frequency of use of other reagents. PTT-A (n=6) and Actin FSL (n=5) were frequently used in the US/Canada and Actin FS (n=6) and Pathromtin SL (n=6) in Europe/Israel. Regional differences in the choice of assay, with US/Canada more likely to use assays that did not accurately measure BAY 94-9027, affected the ability of laboratories to accurately measure FVIII activity in the spiked samples (Figure). In part 1 of the study, regional differences in median recovery of BAY 94-9027 were seen among laboratories when using their own in-house one-stage assays. These differences were not seen in part 2 of the study when laboratories used provided reagents (SynthASil and Pathromtin SL), despite using the same instrumentation as used in part 1. Regional differences in part 1 might have been exacerbated by use of less common, and potentially inappropriate, one-stage assay reagents, affecting the accuracy of BAY 94-9027 measurement. Chromogenic assay use was more common in Europe/Israel (n=11) than US/Canada (n=5). No regional differences in chromogenic assay results were observed.
Conclusions: Most clinical laboratories were able to accurately measure BAY 94-9027 activity using their in-house assays. However, regional differences in the accurate measurement of BAY 94-9027 activity were influenced by regional choice of one-stage reagents used in part 1 of the study; the activity and accuracy differences were eliminated when all laboratories used the same provided one-stage reagents with their in-house instruments (part 2 of study). Therefore, standardization of laboratory procedures and use of newer, more accurate one-stage assay reagents should improve measurement of FVIII activity with EHL products.
Castellone:Bayer: Membership on an entity's Board of Directors or advisory committees; Wilmer-Hale: Consultancy. Church:Bayer: Employment. Leong:Bayer: Employment. Kitchen:Bayer: Consultancy, Other: travel reimbursement, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal